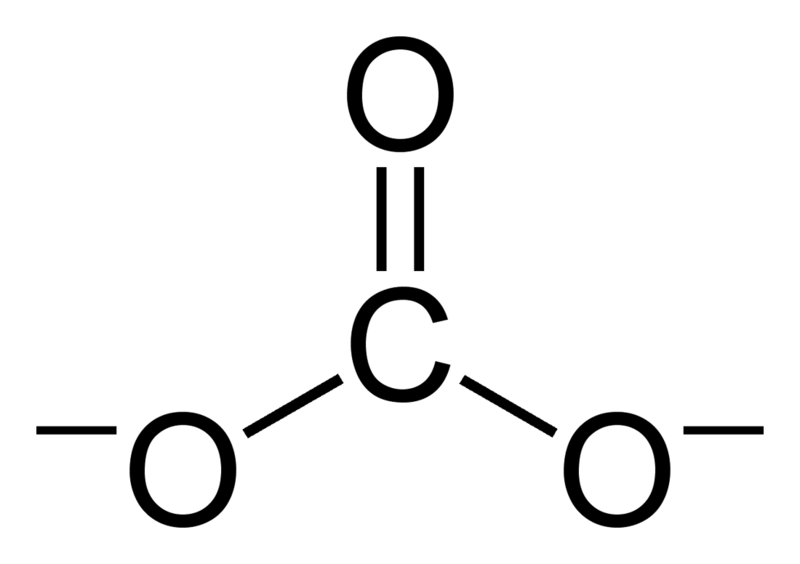
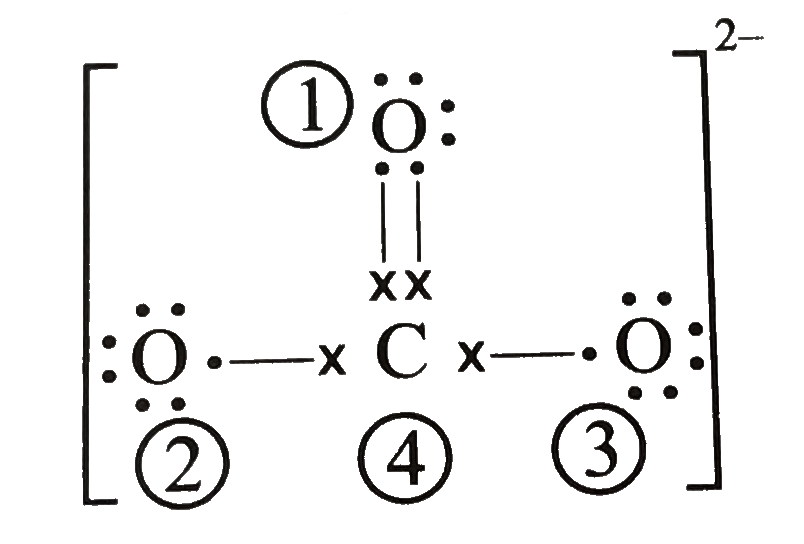
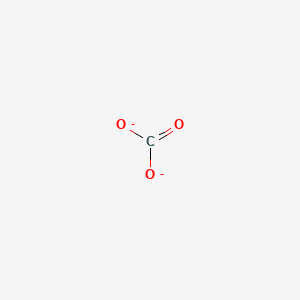
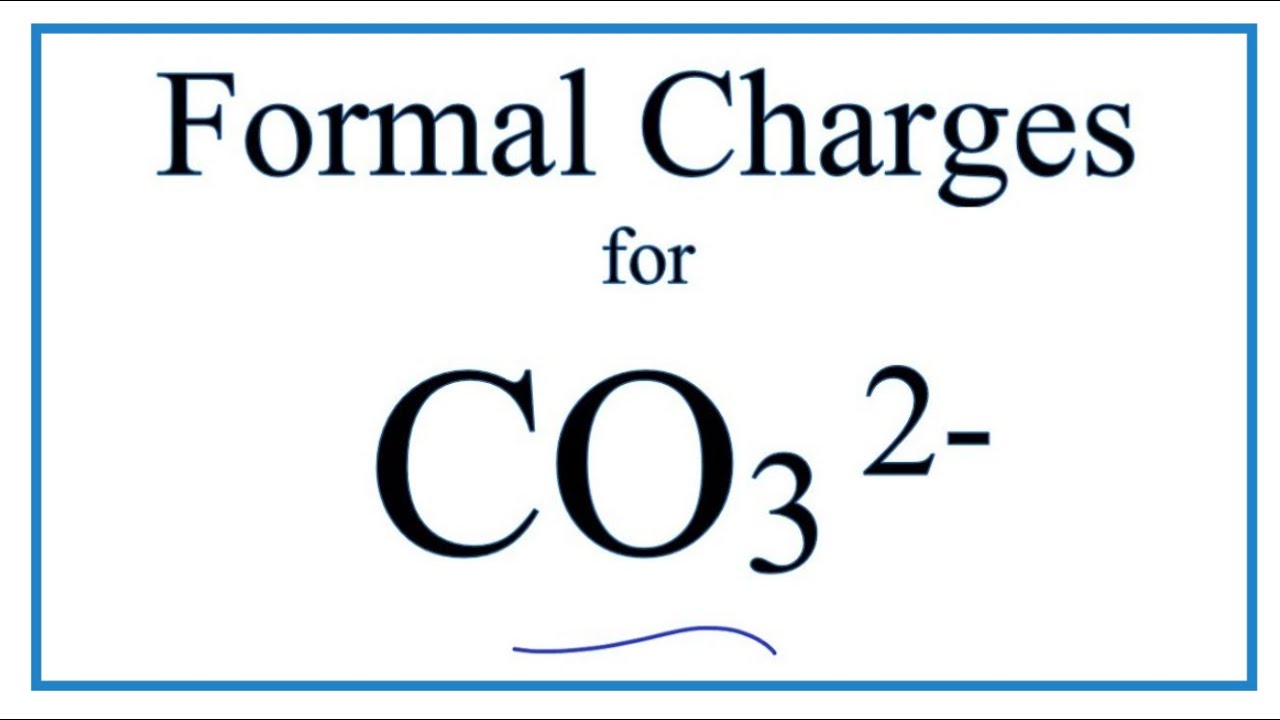Regarding the carbonate ion, CO32-, which of the following statements is false? a. it has resonance b. it has formal charge only on its O atoms c. it has 24 valence electrons
Negatively charged components of oil (a) detached from the positive... | Download Scientific Diagram
![Consider the resonance structures for the carbonate ion.[{Image src='charge5986180229036662068.jpg' alt='charge' caption=''}] | Homework.Study.com Consider the resonance structures for the carbonate ion.[{Image src='charge5986180229036662068.jpg' alt='charge' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/charge5986180229036662068.jpg)
Consider the resonance structures for the carbonate ion.[{Image src='charge5986180229036662068.jpg' alt='charge' caption=''}] | Homework.Study.com

Here is the example in video form.. We're asked to write the formula for calcium carbonate Write the formula for calcium carbonate Ca 2+ CO 3 2– +2 –2. - ppt download

What are polyatomic ions? n Examples: n CO 3 2- = carbonate. n CO 2 2- = carbonite. n PO 4 3- = phosphate n PO 3 3- = phosphite Polyatomic ions are ions. - ppt download
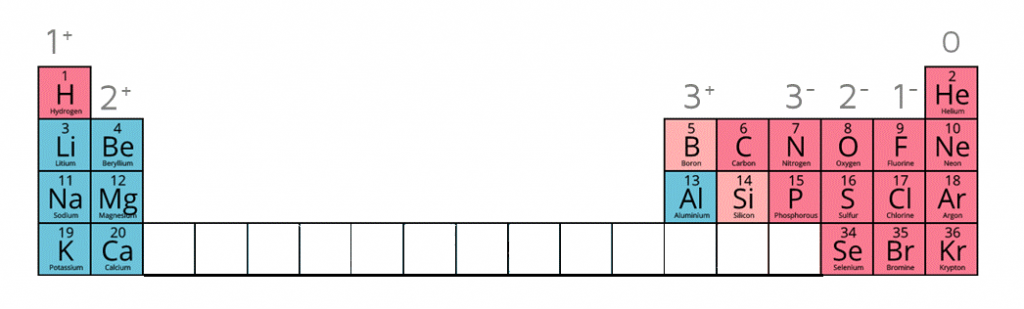
1:38 know the charges of these ions: metals in Groups 1, 2 and 3, non-metals in Groups 5, 6 and 7, Ag⁺, Cu²⁺, Fe²⁺, Fe³⁺, Pb²⁺, Zn²⁺, hydrogen (H⁺), hydroxide (OH⁻), ammonium (