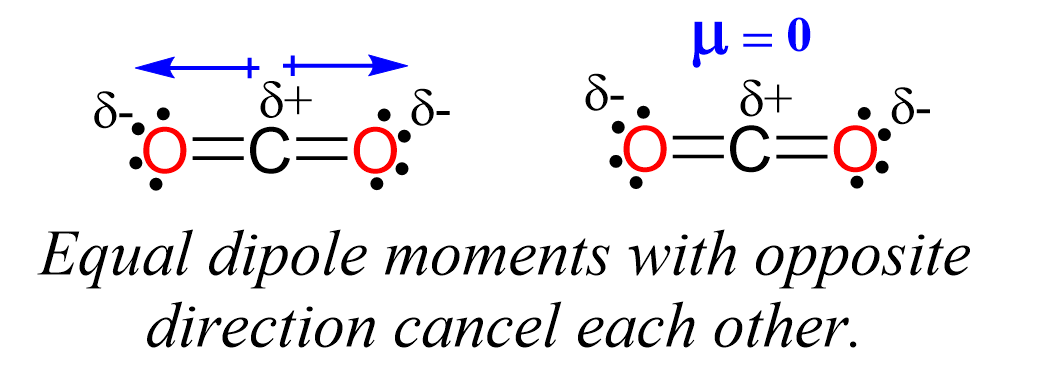
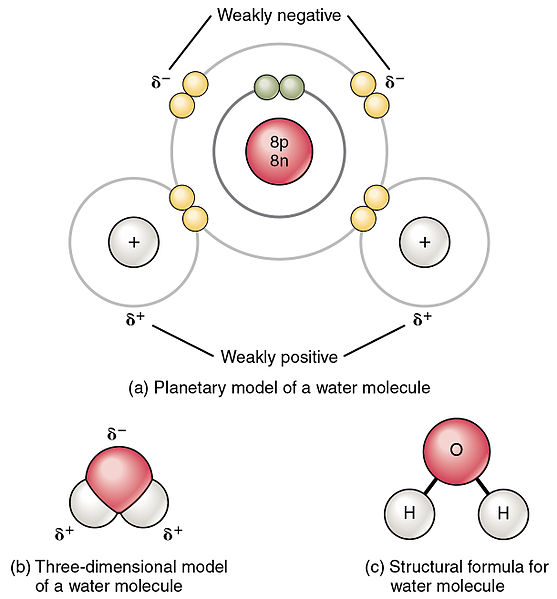
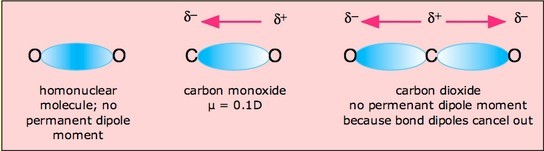
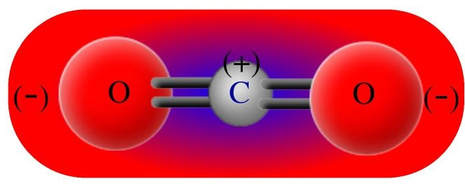
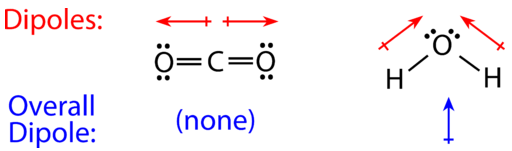
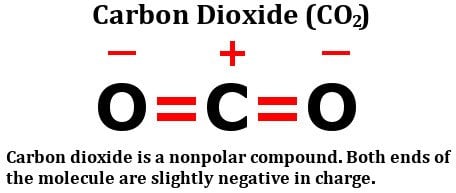Carbon dioxide has two polar bonds, but it is a non-polar molecule. Who can explain this further? - Quora

Draw the Lewis structure for CO2 and state its molecular geometry. Is it polar or nonpolar? | Homework.Study.com

Describe the molecule CO2 in terms of polarity. a. A polar molecule made from nonpolar covalent bonds b. A non-polar molecule made from polar covalent bonds c. A non-polar molecule made from

Figure 2 from Polarity Effect in DC Breakdown Voltage Characteristics of Pressurized Carbon Dioxide up to Supercritical Conditions | Semantic Scholar
Carbon dioxide has two polar bonds, but it is a non-polar molecule. Who can explain this further? - Quora