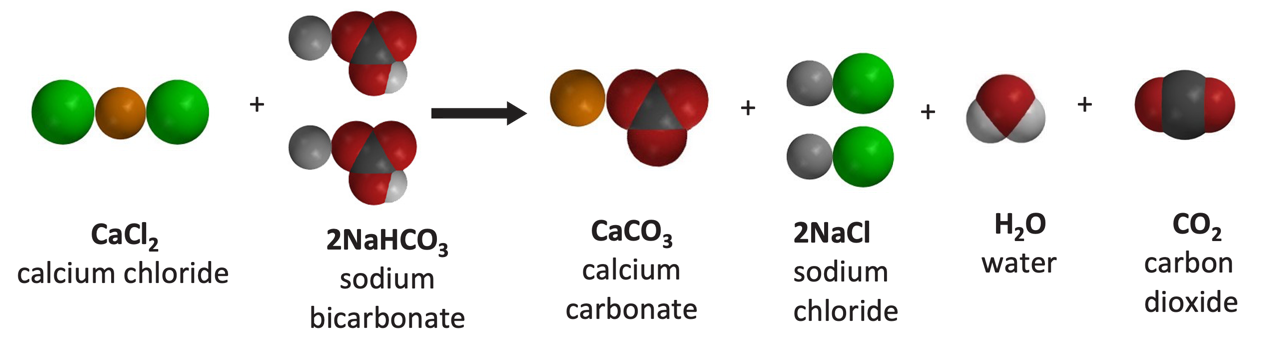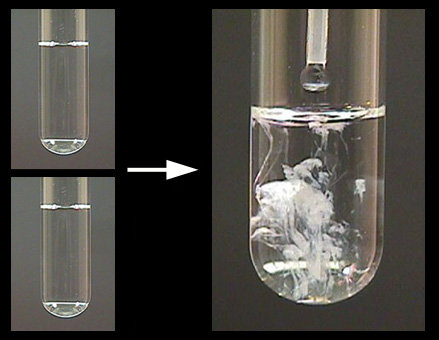
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic

What precipitate will form when aqueous solutions of sodium carbonate calcium Na_2CO_3 and calcium chloride CaCl_2 are mixed? | Socratic

Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution

Forests | Free Full-Text | Hot Compression of Calcium Chloride and Sodium Carbonate Modifies Wood for Tsoongiodendron odorum

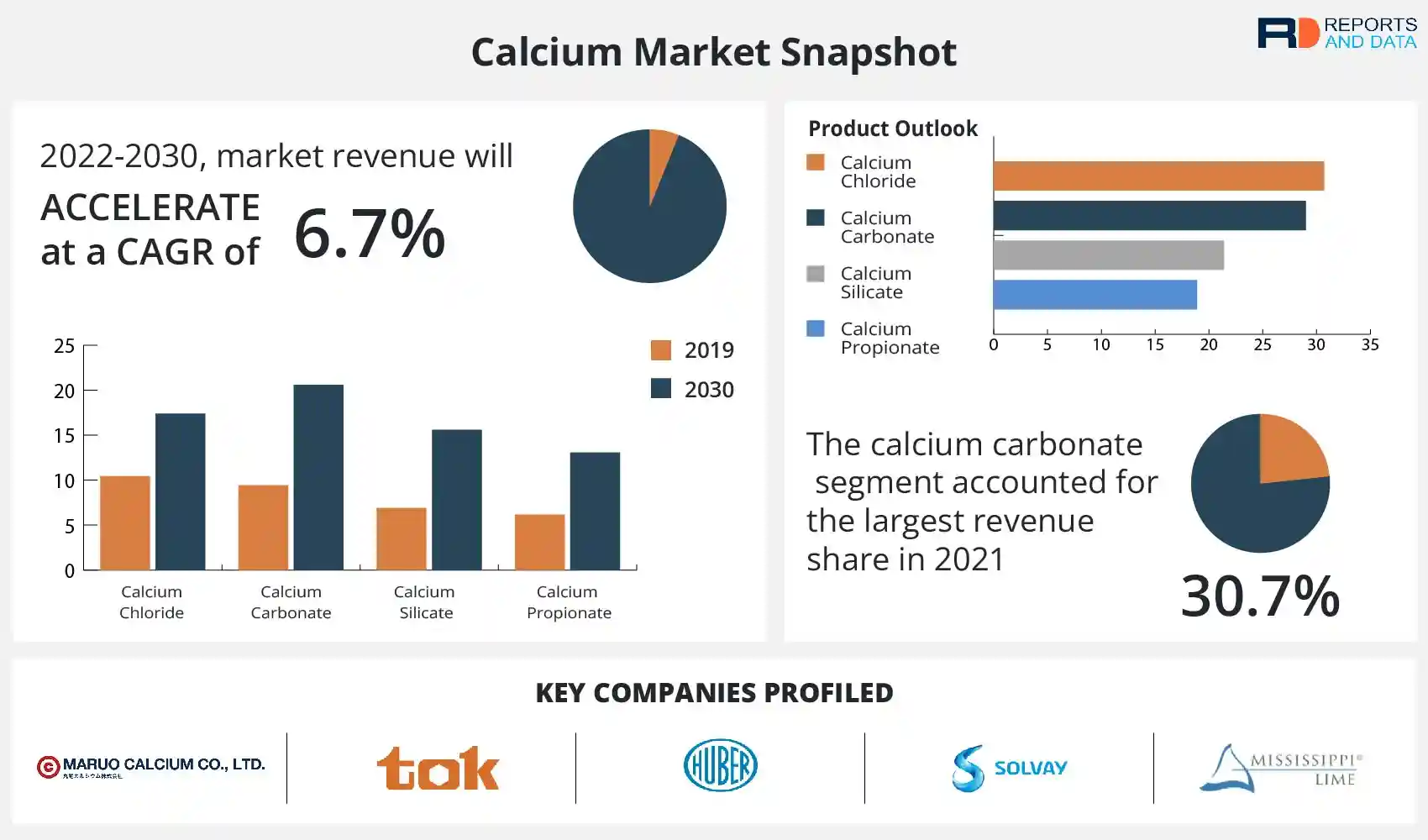
Difference Between Calcium Chloride and Potassium Chloride | Compare the Difference Between Similar Terms









:max_bytes(150000):strip_icc()/calcium-supplements-p2-190470-011-ebed6239876c43f6a071920a617f8a87-38c5a9c6ec6b4f3182f4d2d146fc84d3.png)