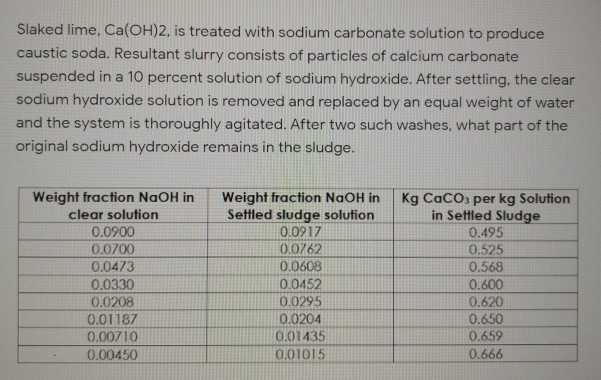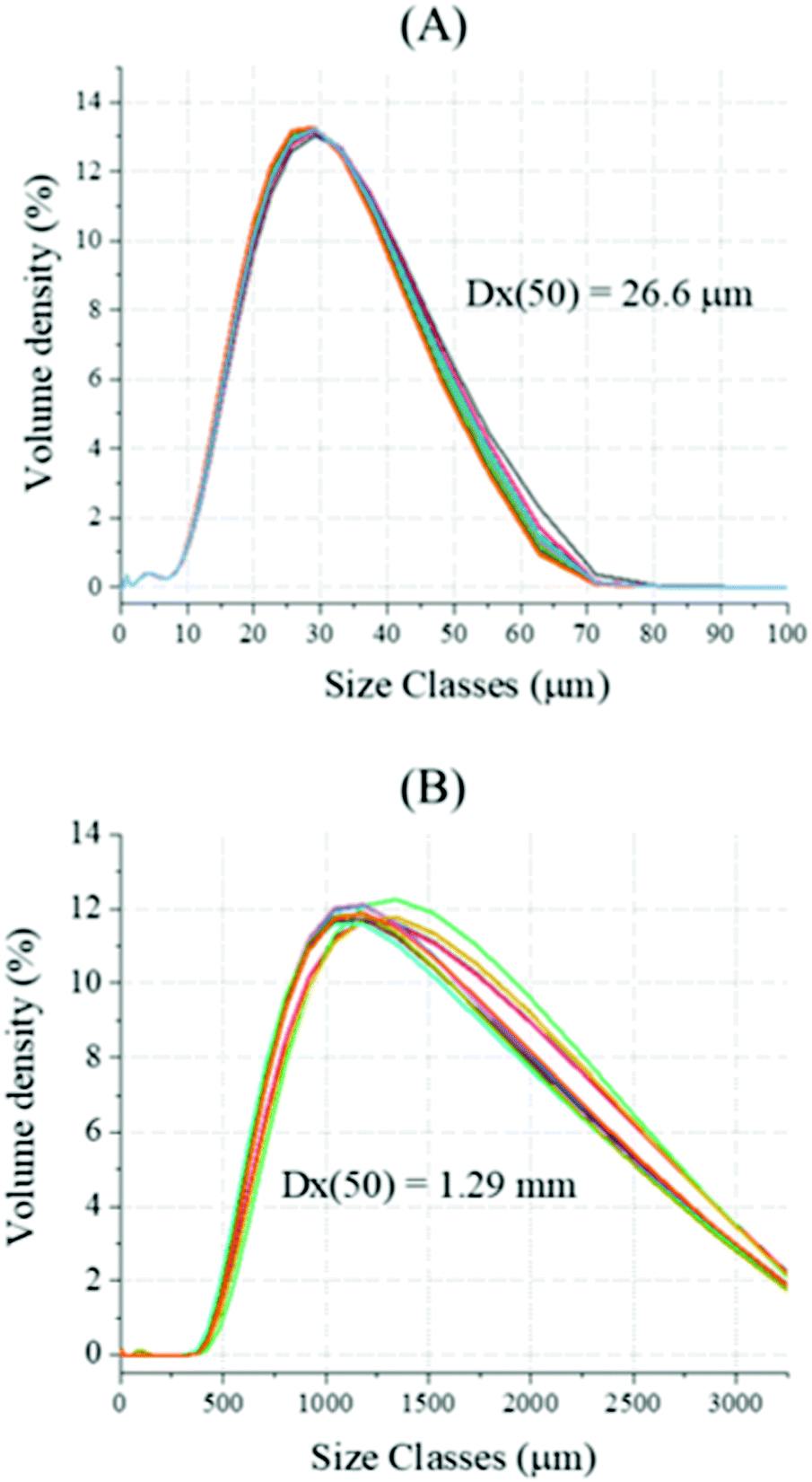
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/D2CP01412B
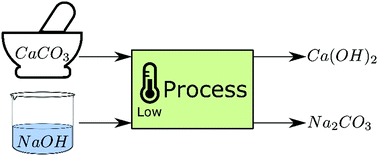
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)

Caustic Soda Flakes 99% Water Treatment Sodium Hydroxide Factory Price - China Potassium Hydroxide, Caustic Soda Factories | Made-in-China.com
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio

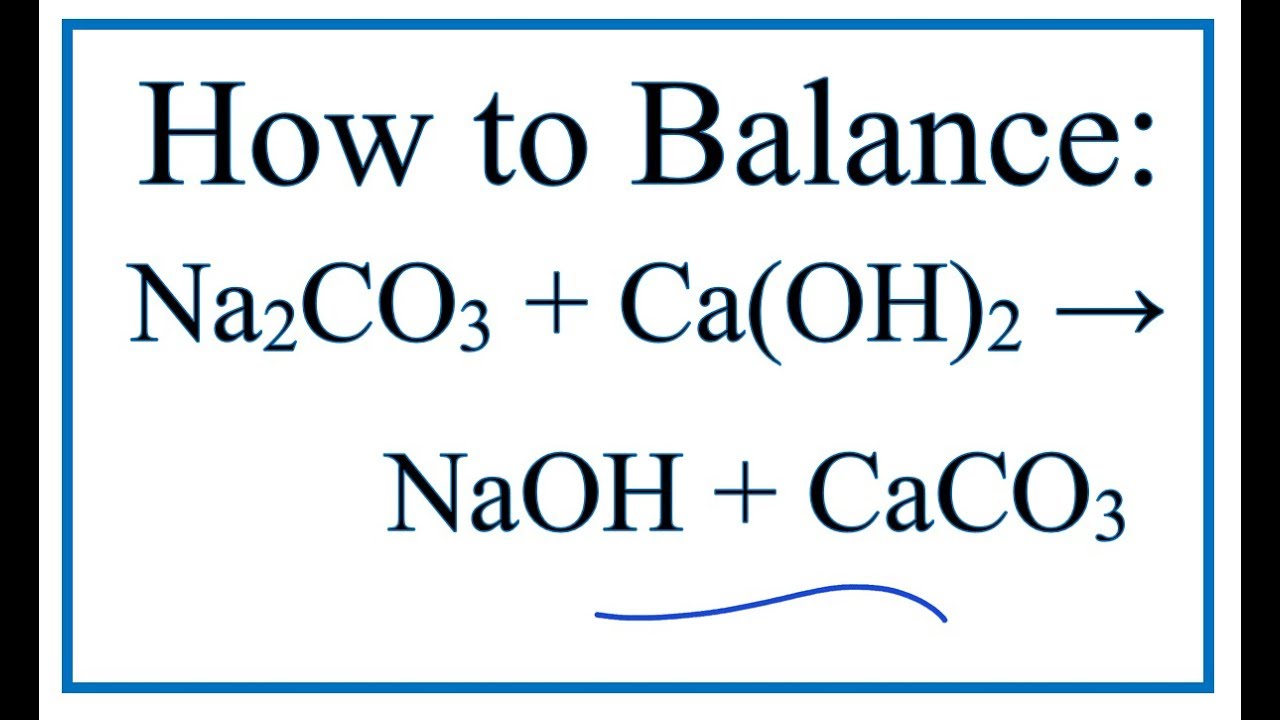
SOLVED: If 3.5 moles of sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2CO3 + Ca(OH)2 NaOH + CaCO3
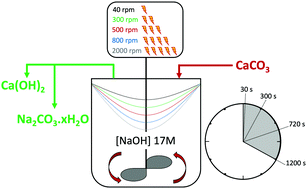
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing)
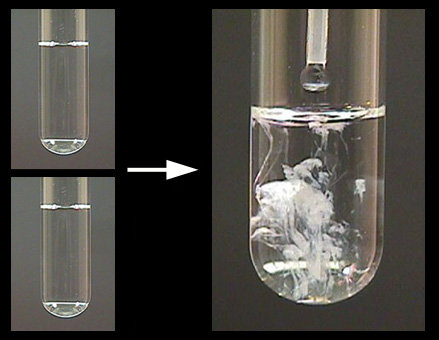
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio

The Effect of Variation Concentration Sodium Hydroxide (NaOH) on the Structure of Calcium Carbonate (CaCO3) Based on Natural Sand | Scientific.Net